Man with Artificial Titanium Heart Survives Over 100 Days
BiVACOR, an Australian company, has successfully implanted its Total Artificial Heart (TAH) into a patient with heart disease. The patient survived for 100 days with the device, which features a single moving part which is a magnetically levitated rotor that pumps blood to both the body and lungs. Details below 👇
An Australian man in his 40s lived for 100 days with BiVACOR's artificial titanium heart while waiting for a donor transplant.

He received the implant during surgery at St. Vincent’s Hospital Sydney in November. In February, he became the first person worldwide to leave the hospital with the device, which kept him alive until a donor heart was made available earlier this month.
The patient, who had severe heart failure, is reportedly recovering well after receiving the implant.
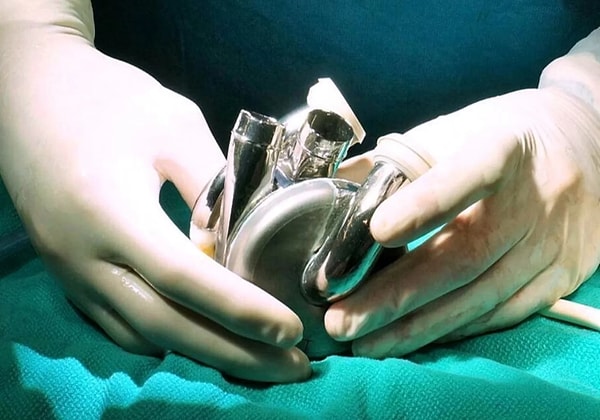
This marks a significant achievement in the development of BiVACOR's Total Artificial Heart (TAH), which has shown potential as a long-term solution for people suffering from heart failure. However, the device is still in clinical trials and has not yet been approved for general use. Its ability to sustain life for an extended period is considered a milestone, indicating the device’s potential to address heart failure more effectively in the future.
BiVACOR, the US-Australian company behind the artificial heart, celebrated the success as a culmination of years of research and development.

Daniel Timms, the Australian bioengineer who invented the device following his father’s death from heart disease, described the accomplishment as “exhilarating.” Timms thanked the patient and his family for their bravery and trust in the technology, noting that their participation would help pave the way for the use of this life-saving technology in future patients.
The BiVACOR Total Artificial Heart (TAH) is constructed from titanium and features a single moving part which is a magnetically levitated rotor.

This rotor is held in place by magnets, eliminating the need for valves or mechanical bearings that could wear out over time. The TAH functions by pumping blood to both the body and lungs, replacing the two ventricles of a failing heart. Its unique design and lack of moving parts that are prone to wear makes it a promising option for patients with severe heart failure.
Cardiovascular diseases are the leading cause of death worldwide, with around 18 million people dying each year from heart-related issues.
The BiVACOR TAH offers a potential solution to address the growing number of people waiting for heart transplants. In the United States, approximately 3,500 heart transplants were performed in 2024, while over 4,400 people joined the waiting list that year. The development of this artificial heart could alleviate the strain on organ donation systems by providing a reliable alternative for patients in urgent need of a new heart.
Artificial hearts like BiVACOR's could become a common alternative for patients who are unable to wait for a donor heart or when a suitable donor is unavailable.

The device has already been tested in the US as part of the Food and Drug Administration's Early Feasibility Study, where it was successfully implanted in five patients. The trial is expected to expand to 15 patients, and it is hoped that these trials will provide further data to support the commercialization of the device.
Keşfet ile ziyaret ettiğin tüm kategorileri tek akışta gör!




Send Comment